Cardiovascular
Study Experience
Successfully accelerating research across therapeutic areas and geographies.
Experience takes many forms. It includes a decade-long track record of successfully recruiting, enrolling and supporting study conduct in every corner of the country, while our highly accomplished Principal Investigators bring expert oversight to virtual trials across the widest range of therapeutic areas and indications.
Therapeutic Areas / Indications
Clinical trial recruitment and conduct can present a much different set of challenges based on the study’s therapeutic area and indication. That’s why finding a clinical research partner with experience supporting your area of study is critical to your success. Here’s a look at just some of our therapeutic expertise.
In-Home Services
Bringing clinical research to new patient populations requires expert investigator oversight of experienced mobile healthcare professionals skilled in providing high quality study conduct in the convenience of the patient’s home.
Our Nurses carefully assess each participant’s home environment to help assess protocol eligibility and the need for any specialized care to assure protocol compliance. That includes Assisted Living Facilities, Group Homes, and more.
- Broadly licensed:
Our Nurses and Investigators are broadly licensed across all 50 U.S. states, allowing us to meet participants wherever they are. Whether they're on vacation or traveling, we ensure their visits are maintained within their study windows. - Pediatric nursing capabilities:
We conduct pediatric visits using our specialized Science 37 Pediatric Nurse Network. - Electronic collection and documentation:
All Source Data is accessible for immediate Quality Control (QC) by a Clinical Research Coordinator (CRC) and Investigators.
- Concomitant medication review
(direct visualization of all medications in the home) - Facilitating identification of AE/SAE/protocol deviation
and reporting findings to Investigator and Study Team in real-time - Vital sign collection
(height, weight, waist circumference, BMI calculation, BP, pulse, pulse OX, temperature, respiratory rate) - Point-of-care testing
(blood glucose monitoring, home pregnancy testing, urine dipstick testing, rapid or PCR COVID-19 testing). - Echocardiogram
(local and in-home) - EEG
(with vendor) - 12-Lead ECG
single and triplicate
(preferably with our Spaulding device, able to use Sponsor requested devices as well) - Investigator-guided telemedicine physical exams
(our Nurses act as the eyes, hands, and ears of the Physician) - Protocol-specific participant and/or caregiver education and training
(IMP self-administration training, eDiary/Diary, diet diary education and collection, self-assessment training as required by protocol — BP collection, PROs, etc) - Investigator-guided specialized exams:
- basic neurological
- in-depth neurological
- SLEDAI/ESSDAI
- 28-joint count
- UPDRS
- Other disease and protocol-specific exams - Facilitating cognitive assessments
- Facilitate neuropsych assessments
(with rater) - In-home spirometry and pulmonary function testing
- Specialized protocol-specific assessments
Nurses are trained & tested for competency
(e.g. 6-Minute Walk Test) - High-resolution digital photography
(full body/partial/body part for ClinROs, etc). Diary, eDiary, diet diary education and collection - Facilitate COA, ClinRO:
telemedicine-based (observation, interview) - Facilitate ClinRO: e.g. EASI, PASI
(photo-based dermatology assessments) - Facilitate ClinRO:
hands-on requirements
(joint assessments by Nurses that are Investigator instructed and observed via telemedicine) - Facilitate COA:
PROs, ObsRO
- Sourcing, transporting and handling of dry ice.
- Laboratory sampling, processing and/or specialized processing, packaging, shipping/transporting
(all Nurses are IATA Certified). - Serial PK/PD/PG collection.
- Specimen collection
(blood, stool, urine, saliva, buccal, nasal, sputum, skin/wound secretions). - Standardized temperature monitoring of lab specimens
(per protocol/lab manual requirements). - Safe handling and shipping of sharps for incineration.
- Receiving IMP shipment from courier
(e.g. in the case of studies with a blinded and unblinded Nurse). - IMP administration
(oral, inhaled, topical, intramuscular, infusion, subcutaneous - IMP accountability, compliance, and reconciliation.
- Standardized temperature monitoring of IMP
(per protocol/pharm manual requirements).
Active Studies Snapshots
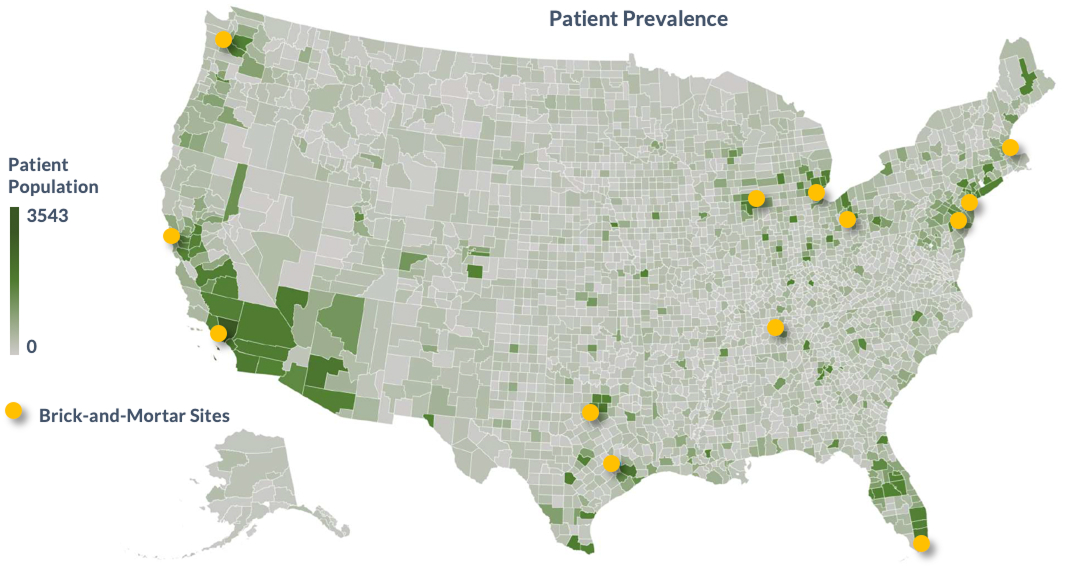
To help you visualize the impact of our patient recruitment and virtual study conduct capabilities, see how we’re performing in a number of active studies.
Sample Protocol: Patient Population Access Comparison
| Science 37 | 133,670 | 100% |
|---|---|---|
| Brick-and-Mortar Sites | 14,703 | 11% |
| Population unseen without Science 37 | 118,967 | 89% |