Direct-to-Patient Site
Science 37 brings your study directly to patients, delivering high-quality care and assessments in the comfort of their own homes.
Limited Patient Access is Stalling Enrollment
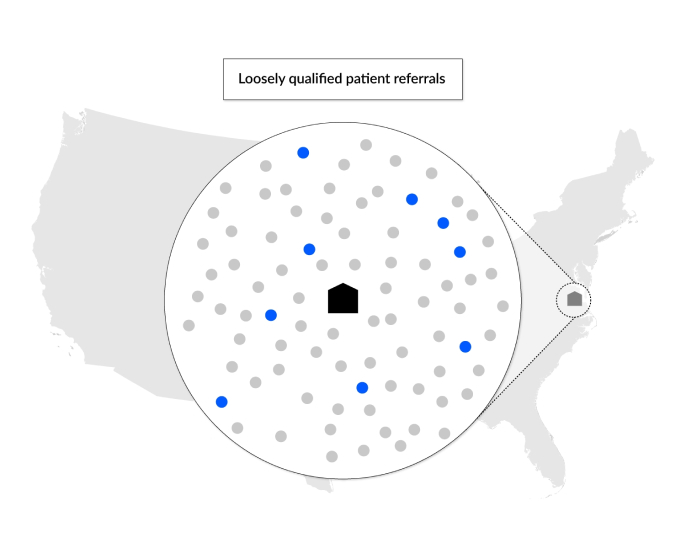
Clinical research has a patient access problem
In the United States, only 8% of the patient population has access to traditional clinical trials. This leaves a large gap in research participation, as many disease states and patient locations don’t align with the physical sites where trials are conducted. Geographic barriers often exclude entire regions from participating in critical research.
Key barriers to enrollment
Geographic Distance: Patients often live far from research sites.
Transportation Challenges: Many lack reliable transportation options.
Time Off Work: Patients may be unable to take the necessary time off to participate.
These obstacles contribute to under-enrolled studies, hindering progress and excluding diverse patient populations.
Impact on Sponsors
Stalled enrollment has serious consequences for clinical researchers:
Longer Timelines: Under-enrolled trials take more time to complete.
- 80% of trials are delayed due to enrollment
- 55% of trials terminate due to failure to achieve enrollment goals
Higher Costs: Delays lead to increased operational expenses.
- Trial delays cost sponsors $600,000—$8 million for each day that a trial delays
Approval Delays: Regulatory approvals are postponed, slowing down time-to-market for life-saving treatments.
Clinical research has a patient access problem
In the United States, only 8% of the patient population has access to traditional clinical trials. This leaves a large gap in research participation, as many disease states and patient locations don’t align with the physical sites where trials are conducted. Geographic barriers often exclude entire regions from participating in critical research.
Key barriers to enrollment
Geographic Distance: Patients often live far from research sites.
Transportation Challenges: Many lack reliable transportation options.
Time Off Work: Patients may be unable to take the necessary time off to participate.
These obstacles contribute to under-enrolled studies, hindering progress and excluding diverse patient populations.
Impact on Sponsors
Stalled enrollment has serious consequences for clinical researchers:
Longer Timelines: Under-enrolled trials take more time to complete.
- 80% of trials are delayed due to enrollment
- 55% of trials terminate due to failure to achieve enrollment goals
Higher Costs: Delays lead to increased operational expenses.
- Trial delays cost sponsors $600,000—$8 million for each day that a trial delays
Approval Delays: Regulatory approvals are postponed, slowing down time-to-market for life-saving treatments.

Without addressing these access barriers, sponsors risk missing critical milestones, driving up costs, and losing valuable time, ultimately threatening the success of their programs and their competitive standing in the marketplace.
How Science 37 Overcomes Enrollment Barriers
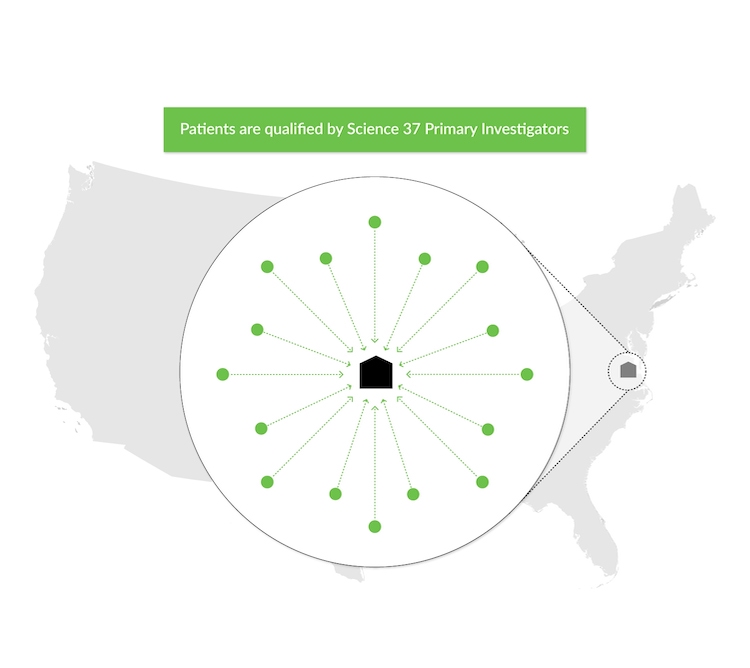
The Science 37 Direct-to-Patient Site
Science 37 addresses the patient access challenge with our Direct-to-Patient Site solution, a direct-to-patient research site that expands clinical trials beyond the geographic limits of traditional sites.
The Science 37 Direct-to-Patient Site
Science 37 addresses the patient access challenge with our Direct-to-Patient Site solution, a direct-to-patient research site that expands clinical trials beyond the geographic limits of traditional sites.


The site that visits the patient
Our Direct-to-Patient Site brings high-quality care and assessments directly to participants—no matter where they are. Whether they live in rural areas or urban centers, patients can participate in clinical trials from the comfort of their homes.

The site that visits the patient
Our Direct-to-Patient Site brings high-quality care and assessments directly to participants—no matter where they are. Whether they live in rural areas or urban centers, patients can participate in clinical trials from the comfort of their homes.
Expanding access and enrollment nationwide
By eliminating geographic barriers, Science 37 is expanding trial access from just 8% of the U.S. population to 100%. This not only accelerates enrollment but ensures that your study reaches its full potential, unlocking access to more diverse and representative patient populations.
Expanding access and enrollment nationwide
By eliminating geographic barriers, Science 37 is expanding trial access from just 8% of the U.S. population to 100%. This not only accelerates enrollment but ensures that your study reaches its full potential, unlocking access to more diverse and representative patient populations.

Ensuring Broad Reach
and Efficient Study Execution
With 50-state licensure, our medical teams manage studies nationwide, ensuring broad patient access. This nationwide capability facilitates seamless enrollment regardless of the participant’s location.
We simplify the approval process by consolidating regulatory requirements into a single 1572 and IRB submission. This streamlined approach reduces complexity, speeding up trial start-up and accelerating the path to First Patient In (FPI).
Study execution from contact to close-out Our solution recruits, enrolls, operationalizes, and manages the entire clinical trial remotely. From patient consent to data collection and assessments, we provide real-time oversight and execution.
Our clinical trials meet the highest quality standards, validated through our successful FDA inspection. This oversight ensures the reliability and integrity required for trustworthy research outcomes.
A Solution Designed Around
Participant Needs
Convenient participation
Science 37 uses remote visits, telemedicine, and at-home support to allow patients to participate in clinical trials from the comfort of their homes. This approach ensures they receive comprehensive care without the need for frequent site visits.
No travel barriers
We eliminate the need for participants to travel by bringing the study directly to them, even in remote locations. This ensures that patients can easily take part in the trial without the burden of long-distance travel, regardless of where they live.
Flexible scheduling
Participants can schedule study visits at times that suit their personal lives, allowing them to engage in trials without disrupting daily routines. Whether facing time constraints or traveling, Science 37's flexibility ensures they can always attend visits.
Flexible scheduling
For immunocompromised patients or those with serious health conditions, at-home visits provide a safer alternative to in-person appointments. This reduces exposure to health risks while delivering personalized care and close monitoring throughout the study.
Continued care wherever participants go
Whether patients relocate or travel during the trial, we ensure continuity of care. Science 37’s adaptable approach allows us to follow participants wherever they are, ensuring they stay engaged and never miss a visit—keeping the study on track.